Selastin Filler

What is it?
The chemical reaction between crosslinking agent and HA occurs through technology development. The crosslinking reaction conditions were optimized. This maximizes the efficiency of cross-linking reactions, resulting in uniform particle size and stable viscoelasticity. A constant and smooth injection force is maintained.ln addition, the toxicity of the BDDE cross-linking agent is maintained at the lowest measurable standard by minimizing the use of a toxic cross-linking agent and performing dialysis process control.

Features of CNB International Products Co., Ltd
Maintain constant viscoelasticity
- Constant viscoelasticity plays an important role in the determination of the procedure and the corrective procedure as intended by the operator. Constant viscoelasticity plays an important role in the determination of the procedure and the corrective procedure as intended by the operator.CNB International Co., Ltd. has standarts five times higher than other companies for research to maintain viscoelasticity and stability. Only a difference of about +/-30 can be considered to be uniform at all times with much higher viscoelasticity than
the permission standard compliance. - The filler of CNB International Co., Ltd. maintains stable viscoelasticity even in recommended storage and overseas transportation conditions.
Maintain constant injection force
- Maintain an injection capacity of 25N or less
- Consideration of maximum convenience for the feeling of use when injecting fillers
Dialysis process management
- 1st Dialysis: Remove NaOH and BDDE using osmotic pressure Replacement of 1M NaCL aqueous solution 4 times a week
- Secondary dialysis: Fit chemical properties of the final product (pH, osmotic pressure) with PBS Buffer, replacement of dialysate 4 times a week
SelastinTM Technology Differentiation and Product Excellence

SelastinTMmaximizes crosslinking with optimized reaction conditions, minimizing the use of crosslinking agents, making it human-friendly and safe.
In addition, we have constructed a product line that maintains uniform, smooth injection power and specific viscoelasticity for each product to suit the area and purpose of use with formulation stability.
This SelastinTM provides a safe, satisfying cosmetic effect fo the patient while maximizing the patient’s willingness to treat.
HA Raw Material Features
1) Safety
- Using the Medical Grade Product
- Hyaluronic acid (H4 raw materials used in (Westin”, are safe as fermented raw materials.
- Non-GMO: Thestrains used in fermentation have been recognized for their safety in compliance with the European Pharmacopeia (EP), Japan Pharmacopeia (JP), a nd Chinese national standards.
- SFDA approval for sodium hyal uronate drug additives (Register No. F20040001,CDE Procedure Document: F20180001497), Sodium Hyaluronate API (Register No. H20113379, CDE Procedure Docu ment: Y20180301650, Registration No. H20133147, CDE Procedure Document: Y20180001413)
2) High Purity
- CNB international’s filler raw material HA quality is much higher than The European Pharmacopoeia standard.
- The management of impurities is much more stringent.
3) Building a high standard production line
- United States DMF
- European Union CEP
- Korea KDMF
- Japan PO
- Pre-registration of raw materials and medicines
- ISO 50301 Energy Management System
- ISO 14001 Environmental Management System, OHSAS 18C0
SelastinTM Technology Differentiation and Product Excellence

SelastinTMmaximizes crosslinking with optimized reaction conditions, minimizing the use of crosslinking agents, making it human-friendly and safe.
In addition, we have constructed a product line that maintains uniform, smooth injection power and specific viscoelasticity for each product to suit the area and purpose of use with formulation stability.
This SelastinTM provides a safe, satisfying cosmetic effect fo the patient while maximizing the patient’s willingness to treat.
Selastin filers
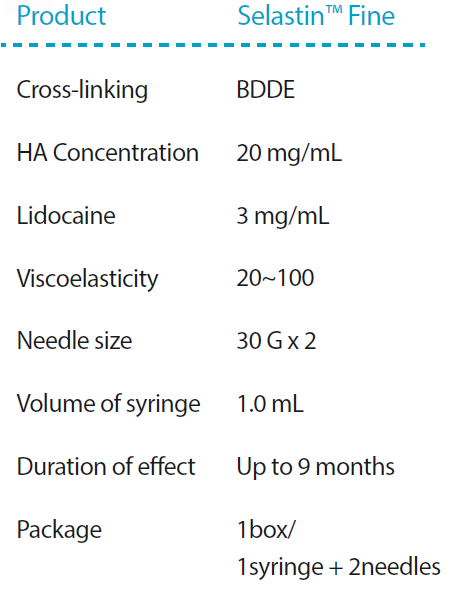
Selastin Fine

Selastin Deep


Selastin Ultra